 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| TSR-H82F3 | Human | Biotinylated Human TSLP R Protein, Fc,Avitag™ |  |
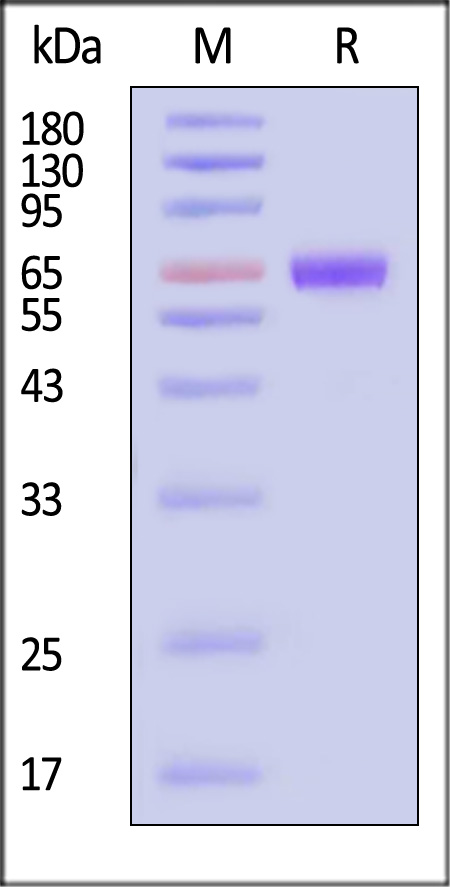
|
|
| TSR-H525a | Human | Human TSLP R Protein, Fc Tag (MALS verified) |  |

|

Captured Human TSLP Protein, His Tag, premium grade (Cat. No. TSP-H52Hb) on CM5 Chip via anti-His antibody, can bind Human TSLP R, Fc Tag (Cat. No. TSR-H525a) with an affinity constant of 1.81 nM as determined in SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Tezepelumab | AMG-157; MEDI-9929 | Approved | Amgen Inc | Tezspire | United States | Asthma | Astrazeneca Ab | 2021-12-17 | Immune System Diseases; Hypersensitivity; Dermatitis, Atopic; Hypersensitivity, Immediate; Bronchial Diseases; Pulmonary Disease, Chronic Obstructive; Sinusitis; Respiratory Hypersensitivity; Asthma; Eosinophilic Esophagitis; Lung Diseases, Obstructive; Lung Diseases; Respiratory Tract Diseases; Nasal Polyps; Nose Diseases; Chronic Urticaria; Anaphylaxis; Carcinoma; Granulomatosis with Polyangiitis | Details |
| Tezepelumab | AMG-157; MEDI-9929 | Approved | Amgen Inc | Tezspire | United States | Asthma | Astrazeneca Ab | 2021-12-17 | Immune System Diseases; Hypersensitivity; Dermatitis, Atopic; Hypersensitivity, Immediate; Bronchial Diseases; Pulmonary Disease, Chronic Obstructive; Sinusitis; Respiratory Hypersensitivity; Asthma; Eosinophilic Esophagitis; Lung Diseases, Obstructive; Lung Diseases; Respiratory Tract Diseases; Nasal Polyps; Nose Diseases; Chronic Urticaria; Anaphylaxis; Carcinoma; Granulomatosis with Polyangiitis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Ecleralimab | CSJ-117; NOV-14 | Phase 2 Clinical | Novartis Pharma Ag | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| WIN-1001X | WIN-1001X | Phase 2 Clinical | Whanin Pharmaceutical Co Ltd | Nerve Degeneration; Parkinson Disease | Details |
| PF-07275315 | PF-07275315 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| Lunsekimig | SAR-443765 | Phase 2 Clinical | Sanofi | Asthma; Inflammation | Details |
| Bosakitug | BSI-045B; TQC-2731; TQC2731 | Phase 2 Clinical | Biosion Inc | Nasal Polyps; Sinusitis; Asthma; Dermatitis, Atopic | Details |
| SHR-1905 | SHR-1905; AIO-001 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Nasal Polyps; Nose Diseases; Respiratory Tract Diseases; Sinusitis; Asthma | Details |
| CM-326(Connaught Biomedical Technology) | CM-326 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Nose Diseases; Nasal Polyps; Asthma; Sinusitis; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details |
| PF-06342674 | RN-168; PF-6342674; PF-06342674; ZB-168 | Phase 1 Clinical | Pfizer Inc | Diabetes Mellitus, Type 1; Multiple Sclerosis | Details |
| HBM-9378 | SKB378; HBM-9378 | Phase 1 Clinical | Harbour Biomed, Sichuan Kelun-Biotech Biopharmaceutical Co Ltd | Asthma | Details |
| IBI-3002 | IBI3002; IBI-3002 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Asthma | Details |
| GB-0895 | GB-0895 | Phase 1 Clinical | Generate Biomedicines Inc | Immune System Diseases; Asthma | Details |
| STSA-1201 | STSA-1201; STSA1201 | Phase 1 Clinical | Staidson(Beijing) Biopharmaceuticals Co Ltd | Asthma | Details |
| LQ043H | LQ043H | Phase 1 Clinical | Shanghai Novamab Biopharmaceuticals Co Ltd | Asthma | Details |
| GR2002 | GR2002 | Phase 1 Clinical | Genrix (Shanghai) Biopharmaceutical Co Ltd | Nasal Polyps; Asthma; Sinusitis; Dermatitis, Atopic | Details |
| AZD-8630 | AZD-8630; AMG-104 | Phase 1 Clinical | Astrazeneca Plc, Amgen Inc | Asthma | Details |
| QX-008N | QX008N; QX-008N | Phase 1 Clinical | Qyuns Therapeutics Co Ltd | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| BD-9 | BD-9 | Biolojic Design Inc | Details | ||
| Ecleralimab | CSJ-117; NOV-14 | Phase 2 Clinical | Novartis Pharma Ag | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| WIN-1001X | WIN-1001X | Phase 2 Clinical | Whanin Pharmaceutical Co Ltd | Nerve Degeneration; Parkinson Disease | Details |
| PF-07275315 | PF-07275315 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| Lunsekimig | SAR-443765 | Phase 2 Clinical | Sanofi | Asthma; Inflammation | Details |
| Bosakitug | BSI-045B; TQC-2731; TQC2731 | Phase 2 Clinical | Biosion Inc | Nasal Polyps; Sinusitis; Asthma; Dermatitis, Atopic | Details |
| SHR-1905 | SHR-1905; AIO-001 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Nasal Polyps; Nose Diseases; Respiratory Tract Diseases; Sinusitis; Asthma | Details |
| CM-326(Connaught Biomedical Technology) | CM-326 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Nose Diseases; Nasal Polyps; Asthma; Sinusitis; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details |
| PF-06342674 | RN-168; PF-6342674; PF-06342674; ZB-168 | Phase 1 Clinical | Pfizer Inc | Diabetes Mellitus, Type 1; Multiple Sclerosis | Details |
| HBM-9378 | SKB378; HBM-9378 | Phase 1 Clinical | Harbour Biomed, Sichuan Kelun-Biotech Biopharmaceutical Co Ltd | Asthma | Details |
| IBI-3002 | IBI3002; IBI-3002 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Asthma | Details |
| GB-0895 | GB-0895 | Phase 1 Clinical | Generate Biomedicines Inc | Immune System Diseases; Asthma | Details |
| STSA-1201 | STSA-1201; STSA1201 | Phase 1 Clinical | Staidson(Beijing) Biopharmaceuticals Co Ltd | Asthma | Details |
| LQ043H | LQ043H | Phase 1 Clinical | Shanghai Novamab Biopharmaceuticals Co Ltd | Asthma | Details |
| GR2002 | GR2002 | Phase 1 Clinical | Genrix (Shanghai) Biopharmaceutical Co Ltd | Nasal Polyps; Asthma; Sinusitis; Dermatitis, Atopic | Details |
| AZD-8630 | AZD-8630; AMG-104 | Phase 1 Clinical | Astrazeneca Plc, Amgen Inc | Asthma | Details |
| QX-008N | QX008N; QX-008N | Phase 1 Clinical | Qyuns Therapeutics Co Ltd | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| BD-9 | BD-9 | Biolojic Design Inc | Details |
This web search service is supported by Google Inc.
